
Safety: Evaluating the Safety of Escalating Doses of CBD in Healthy Dogs
Preliminary Investigation of the Safety of Escalating Cannabinoid Doses in Healthy Dogs
Vaughn, J. Kulpa and L. Paulionis
2020
KEY TAKEAWAYS:
- CBD oil administration had little effect on food intake and physical activity
- There was no difference in the adverse effect profile of CBD oil when compared to its control group
- Administration of CBD oil up to a dose of 62mg/kg per day resulted in mild side effects only and these side effects were also detected in the corresponding placebo group
- No moderate or clinically significant adverse effects were reported after administration of CBD oil up to 62mg/kg daily
- The study provides novel data that separates the safety and tolerability of dose escalation of oil formulations predominant in CBD, THC, or CBD + THC (1.5:1)
- There was a clear distinction in the safety profile of the CBD oil when compared to THC or CBD+THC oil, with CBD oil being the most well tolerated
- The study supports the safe and tolerable profile of CBD oil even when administered up to 62mg/kg daily, which is considered a much higher dose than would be anticipated for clinical use
- Normally recommended doses range from 0.5 to 8mg/kg
OBJECTIVES & HYPOTHESIS → Given the growing interest and evidence of the therapeutic properties of CBD in companion animals, there is a need to assess CBD’s safety profile. The study set out to evaluate the safety and tolerability of escalating doses of three cannabis oil formulations, containing predominantly CBD, THC, or CBD and THC (1.5:1) vs placebo in dogs. A secondary objective was to determine blood levels of CBD, THC, and their metabolites at higher dose levels of CBD and THC.
METHODS → The study was a randomized, placebo-controlled, blinded and parallel study that enrolled 20 healthy dogs. The dogs were randomly assigned to one of five treatment groups: CBD-predominant oil, THC-predominant oil, CBD/THC-predominant oil (1.5:1), sunflower (SF) oil placebo, MCT (medium chain triglycerides) oil placebo. Up to 10 escalating doses of the oils were planned for administrations, with at least 3 days separating doses (the daily maximum doses administered were: 62 mg/kg of CBD, 49 mg/kg of THC and 12 mg/kg CBD + 8 mg/kg THC). Two different placebo oils were used since the cannabinoid oils included either SF or MCT oil as solvents. Experienced veterinary technicians and veterinarians conducted the clinical observations and physical assessments and complete blood counts, clinical chemistry, and plasma cannabinoids were also measured to assess safety, tolerability, and the occurrence of adverse events (AEs).
RESULTS → Of the oils tested, the CBD oil had the least effect on food intake and physical activity. In concern to subject discontinuation, 1 dog in the THC oil group and 2 in the CBD/THC group were discontinued from the study due to severe adverse effects (AEs). No dogs were discontinued from the CBD oil or placebo oil groups as a result of AEs. Side effects were reported in all 5 groups, and out of 505 AEs that were registered in total, 104 occurred in the placebo groups, 80 in the CBD group, 206 in the THC group, and 115 in the CBD/THC group. 94.9% of these adverse effects were mild. Focusing on the CBD oil group only, it is worth highlighting that the number and profile of AEs was similar to the MTC placebo oil group. Moreover, there were no moderate AEs in the CBD oil group at any of the doses tested while in its corresponding placebo group, 2 dogs suffered from mild adverse effects. Also, there were no severe or medically significant side effects in the CBD oil group at any of the doses tested.
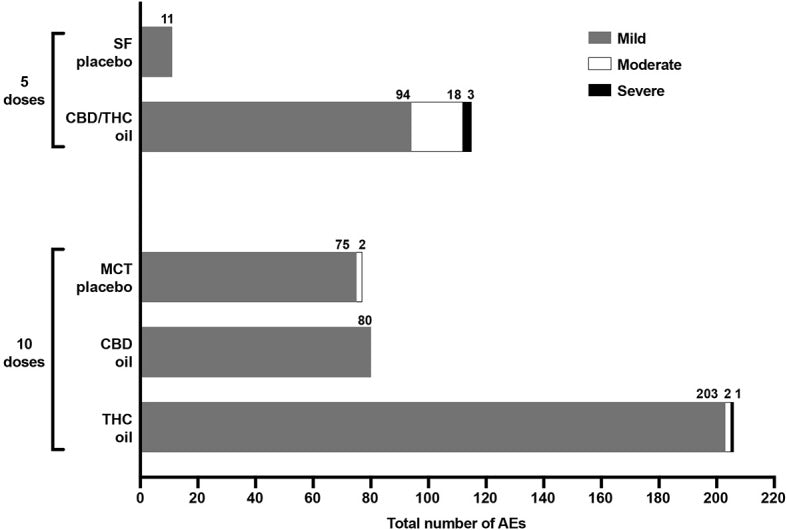
Figure 1: total number and severity of AEs experienced across 5 escalating doses of SF oil placebo and CBD/THC oil and 10 escalating doses of MCT oil placebo, CBD oil and THC oil

Figure 2: Total number of mild AEs per anatomic category. Mild AEs across 10 doses of MCT oil placebo, CBD oil or THC oil.
Moderate AEs occurred in 40% of the subjects across three groups: 2 dogs in the MCT oil group, 2 dogs in the THC oil group and 4 dogs in the CBD/THC oil group. Severe AEs occurred in 15% of the subjects across the THC oil and CBD/THC oil group. Regarding blood and chemical analysis, the only change determined was an increase in ALP in one dog in the CBD oil group (when administered the tenth dose) and one dog in the CBD/THC oil (fifth dose). These changes were not considered clinically significant and when these levels were remeasured 7 days post final dose, there was a downward trend. Plasma levels of CBD, THC, and their metabolites were highly variable between dogs in the same treatment group receiving either the ninth dose of CBD oil or THC oil. Following the ninth dose of the CBD oil, maximum plasma CBD levels achieved across the four dogs in the CBD oil group were comparable to the range in plasma CBD levels achieved across nine dogs following repeated daily CBD dosing (2.5 mg/kg twice daily) for 12 weeks . Based on these results, it appears that a first pass effect through the liver did not eliminate the systemic availability of CBD following its oral ingestion. Furthermore, the fact that quantifiable levels of CBD were observed one week following dose exposure is also interesting, given that CBD has been reported to have a relatively rapid elimination in dogs.
In conclusion, the findings provided by the study support CBD’s safety profile and backs up its potential as a treatment option in veterinary medicine.
